Examples:
(1) geothermal heat flux through the ocean floor = 10-6
cal/cm2/s
(2) Top of atmosphere solar flux = 1300 Joules/m2/s = 1300 W/m2 away
from sun
1. Radiation: We usually think of EM radiation. It carries energy from the sun and returns energy to outer space. But EM transfer inside the ocean is small because the ocean is not very transparent. Energy/momentum transport by internal/surface gravity wave radiation is important but we defer its discussion until these waves have been described. Seismologists are concerned with energy transport by acoustic/shear waves.
2. Advection: motion of fluid carries stuff (such as mass, heat, salt, carbon, etc).
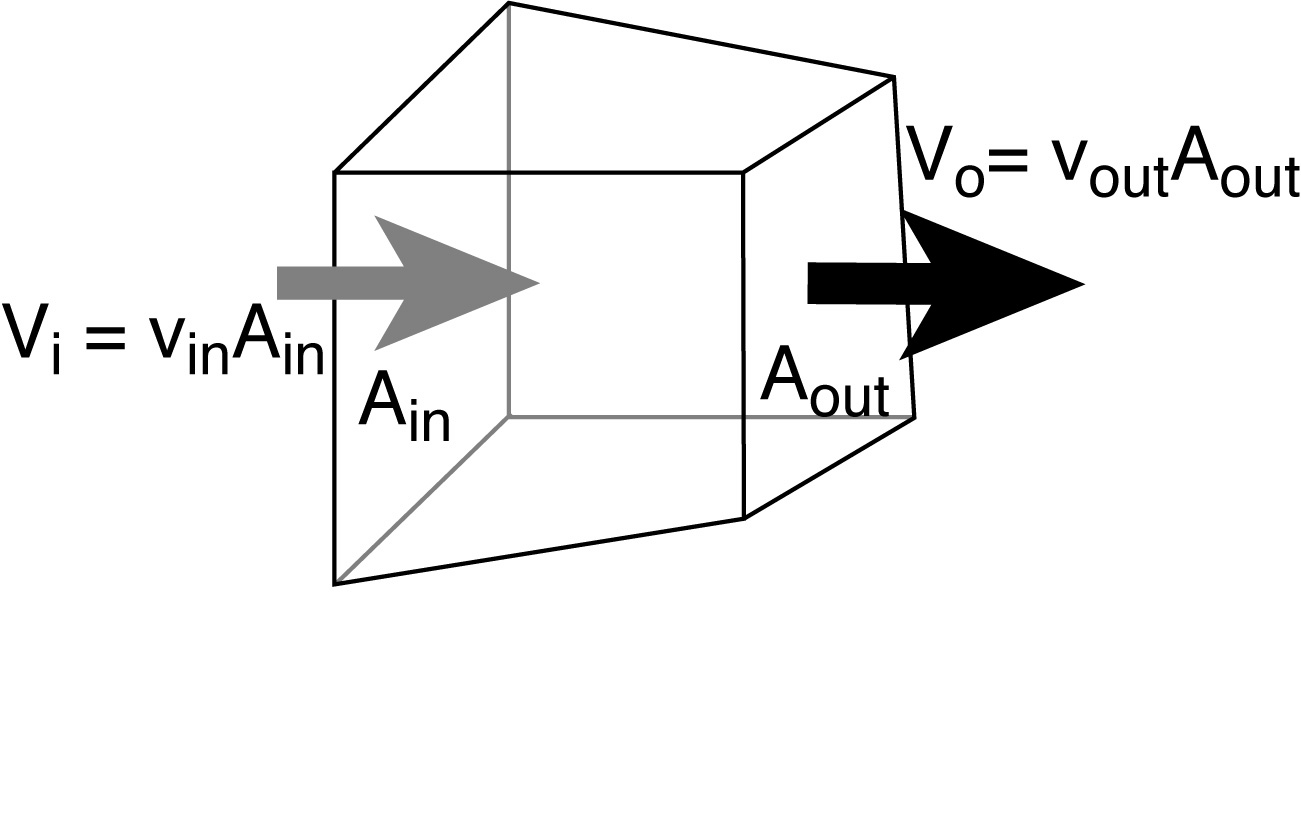
(Advective) flux = velocity times concentration. The units
of concentration are [Concentration] = [stuff/m3] (stuff per unit volume).
Therefore the units of flux are: [ Flux ] = [stuff/m2/sec].
Application in 2D: iso-tracers are streamlines of steady flow i.e. if flow were steady in 2D, measuring one tracer and contouring its isolines would tell us the streamlines (but not the flow speed).
In the ocean, flow is often idealized as steady but is really 3D. Streamlines lie anywhere on isotracer sheets. Measure 2 tracers. Their sheets usually aren't parallel but intersect along a contour. The flow lies in both sheets i.e. along the intersection of the two sheets.
Simple velocity - another application
(figures showing how convergence - i.e. advection - can sharpen gradients)
Transport = flux times area.
Alternatively, think of flux as transport per unit area.
Therefore the units of transport are: [ Transport ] = [stuff / sec].
Volume transport: m3/sec
Mass transport: kg/sec
Salt transport: kg/sec
Freshwater transport: kg/sec
Heat transport: Joules/sec (Watts)
Oxygen, carbon, etc transport: moles/sec
Transports. We often wish to express how much total water or heat or salt or carbon or "stuff" is moving around. Transports are the total flux of whatever property through a given area. The general expression for transports is the (integral of the velocity times the property computed over the area). The area we use for most transports associated with general circulation is a vertical plane (e.g. for horizontal velocities). We can also calculate a vertical transport through a horizontal or isopycnal surface. However, since vertical velocities associated with the general circulation are tiny, vertical transports based on observations (as opposed to model results) are calculated as residuals.
Volume transport: integral of velocity over the area. Units are m3/sec. A typical transport for a major current is on the order of 10 to 100 x 106 m3/sec. We use the unit "Sverdrup" where 1 Sv = 1 x 106 m3/sec.
Mass transport: integral of density*velocity over the area. Units are kg/sec. A typical transport for a major current is order 10 to 100 x 109 kg/sec (since density is roughly 1000 kg/m3).
Heat transport: integral of (heat*velocity) over the area. Units are Watts (e.g. Joules/sec). Heat must be calculated using Kelvin degrees, and potential temperature rather than temperature. Heat transport through a closed area, through which there is no net mass transport, is basically proportional to the temperature difference between water entering and water leaving across the closed boundaries, so degrees Celsius are fine for this practical use. (This can easily be proven by writing the temperature T_k in degrees K in terms of temperature T_c in degrees C and the constant offset between them of 273.15. Then when the heat transport integral is calculated, the portion with the constant 273.15 drops out if the total mass transport through the area is 0.) Net heat transports across various latitude lines which close at boundaries range from -1.0 to 1.0 x 1015 Watts. We often use the unit 1 Petawatt = 1 x 1015 Watts.
Freshwater transport: Salt in the ocean is not gained or lost over the time scales of interest to us. However freshwater is exchanged with the atmosphere and land. A kilogram of seawater consists of a portion of water and a portion of salt. The relative proportions are expressed in the salinity, which is taken for these transport calculations to be (gm salt/kg seawater) or (gm salt/(kg salt + kg freshwater)). Using the salinity measured along a section, we can calculate the freshwater transport across the section. Typical values are order 1 Sv.
Other substance transports (carbon, oxygen, etc etc): If the property is given as a concentration ( gm property/gm seawater), then the transport is integral(concentration*density*velocity) over the area, which yields gm/sec of the substance.
(Figure in class: mass transports in the North Atlantic from Schmitz and McCartney; heat transports for the globe from various sources.)
3. Diffusion (to be covered in first dynamics lecture):
Occurs because molecules are always in random thermal
motion even if material appears at rest. Diffusion needs a
concentration gradient i.e. more stuff one place than somewhere else.
(Materials are in the first dynamics lecture.)